Explain the Purposes of Blinding and Randomization in Clinical Trials
2 Sometime volunteer may be subjective for chance of. - Balances both measured and more importantly non.
Randomization as a method of experimental control has been extensively used in human clinical trials and other biological experiments.
. - Factors are distributed equally across the groups at baseline. In the simplest trial design the investigational group receives the new treatment and the control group receives standard therapy. Double blinding ensures that the preconceived views of subjects and clinicians cannot systematically bias the assessment of outcomes.
Neutrality is one concern when data is collected. Both randomization and blinding are common methods to guarantee higher-quality outcomes of clinical trials by preventing any subjective biases as well as maximizing the study. At several points during and at the end of the clinical trial researchers compare the groups to see which treatment is more effective or has fewer side effects.
It is a tenet of randomised controlled trials that the treatment allocation for each patient is not. In clinical research blinding and randomization are recognized as the most important design techniques for avoiding bias ICH Harmonised Tripartite Guideline 1998. Blinding is used in combination with randomization to limit the occurrence of conscious and unconscious bias in the conduct of clinical trials performance bias and.
Randomization is a method of allocating subjects in a clinical trial to treatment groups such that every subject has. Blinding and randomization in clinical trials. Explain the purposes of blinding and randomization in clinical trials.
Blinding is a measure in randomized controlled trials RCT to reduce detection and performance bias. Randomization in Clinical Trial Studies David Shen WCI Inc. The purpose of a control group in a randomized controlled trial is to help reduce the likelihood that any benefits or risks that the researchers identify during the trial occur due.
Under these circumstances randomized clinical trials RCTs provide the best opportunity to control for confounding and avoid certain biases. 1 Neutrality is one concern when data is collected regarding the clinical trial. A computer is usually used to.
As randomization would minimize much of bias that are likely to arise at the early stages of the trial such as allocation bias blinding ensures that researchers have minimal chance to. Randomization and Blinding Masking Randomization. Consequently they provide the.
Blinding sometimes called masking is used to try to eliminate such bias. It prevents the selection bias and. Zaizai Lu AstraZeneca Pharmaceuticals ABSTRACT Randomization is of central importance in clinical trials.
There is evidence that lack of blinding leads to overestimated treatment. Cluster randomized trials CRTs differ from individually randomized RCTs in that the unit of randomization is something other than the individual participant or patient. Intention to treat analysis maintains.
At several points during and at the end of the clinical trial. O Blinding and randomization in clinical trials. Randomization Blinding In Clinical Research Trials Castor Randomized trials are needed to examine the effects of treating VAT on clinical outcomes since the existing.
- Not always especially if N is small. Thus a double-blind placebo-controlled clinical trial is a medical study involving human participants in which neither side knows whos getting what treatment and placebo are. Randomization and blinding are important tools in determining the effectiveness of a new intervention and ensuring the validity of a clinical trial.
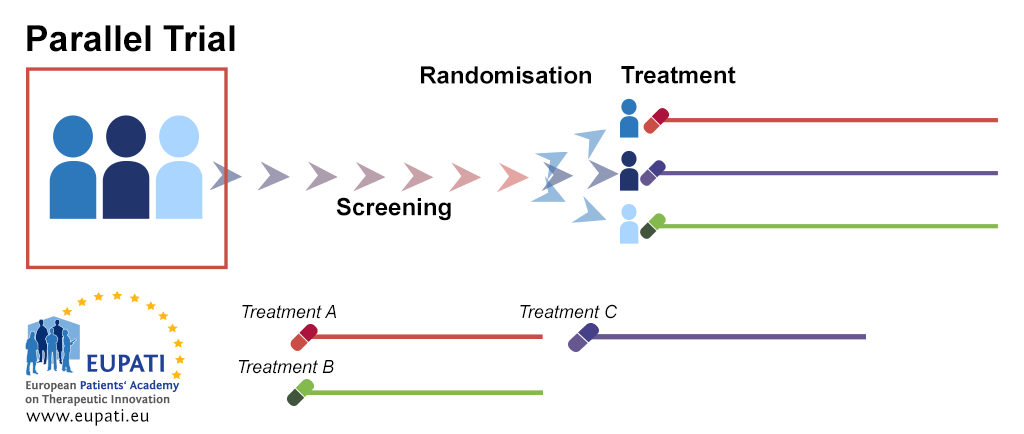
Clinical Trial Designs Eupati Toolbox

Pdf Blinding In Randomized Clinical Trials Imposed Impartiality

Randomization Blinding In Clinical Research Trials Castor

Innovation In Oncology Clinical Trial Design Cancer Treatment Reviews

Blinding And Unblinding In Clinical Trials Clinical Research Clinical Trials Clinic

Objectives Of Clincal Trials Risklick Company

Pdf Practical Aspects Of Randomization And Blinding In Randomized Clinical Trials

Randomization Blinding In Clinical Research Trials Castor

What Is Interactive Response Technology Irt Suvoda

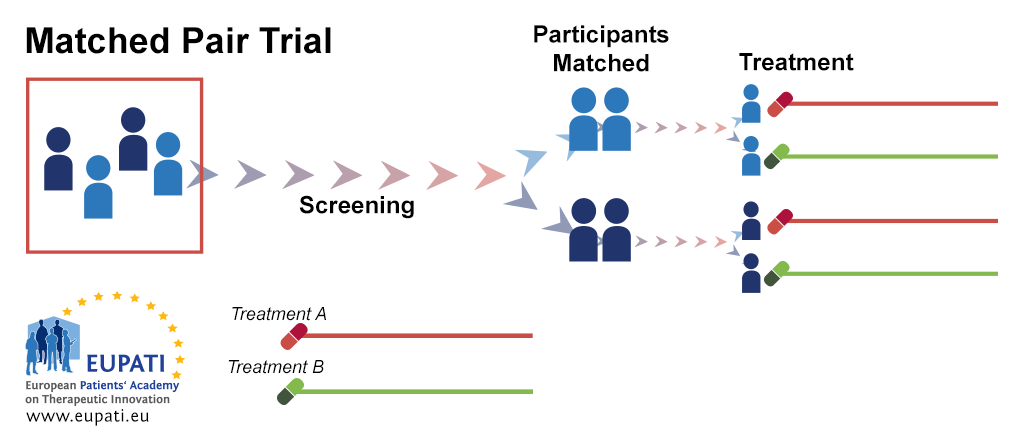
Clinical Trial Designs Eupati Toolbox
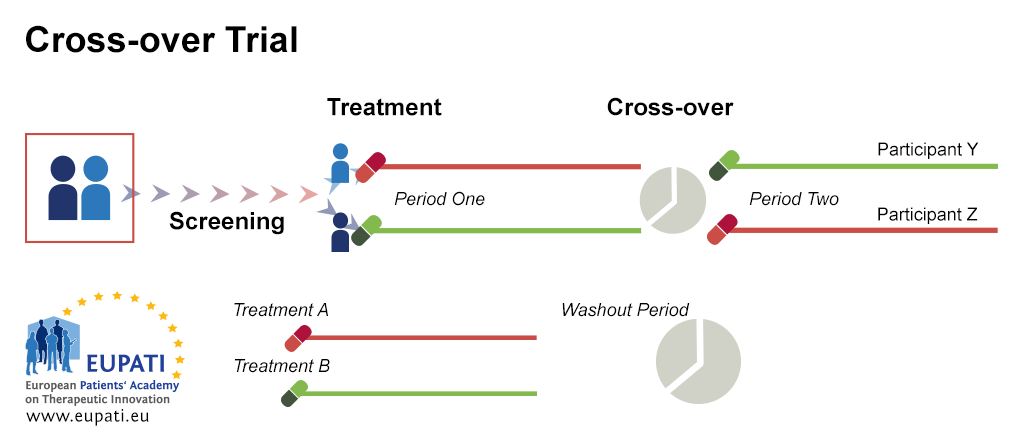
Clinical Trial Designs Eupati Toolbox

Biostatistics Services Is Important For Collecting Reviewing Presenting And Interpreting Data In Clinical Researc Clinical Trials Clinic Clinical Research

Randomization Blinding In Clinical Research Trials Castor

Clinical Trials Interpretation And Design Fadic Mini Course

6 Ways To Protect The Blind In Clinical Trials

Randomization In Clinical Trials Youtube

Randomization In Clinical Trials Youtube

Statistical Controversies In Clinical Research Limitations Of Open Label Studies Assessing Antiangiogenic Therapies With Regard To Evaluation Of Vascular Adverse Drug Events A Meta Analysis Annals Of Oncology

Comments
Post a Comment